The technology underlying the analysis carried out by FetalDNA is recognized by the scientific community as the most advanced in the world, overcoming the limits of tests already on the market.
AN IMPORTANT TEST FOR A SERENE PREGNANCY
FetalDNA is a non-invasive test that poses absolutely no risk for the mother nor the fetus. It is currently the most comprehensive screening test for the study of chromosomal aneuploidies affecting the fetus.
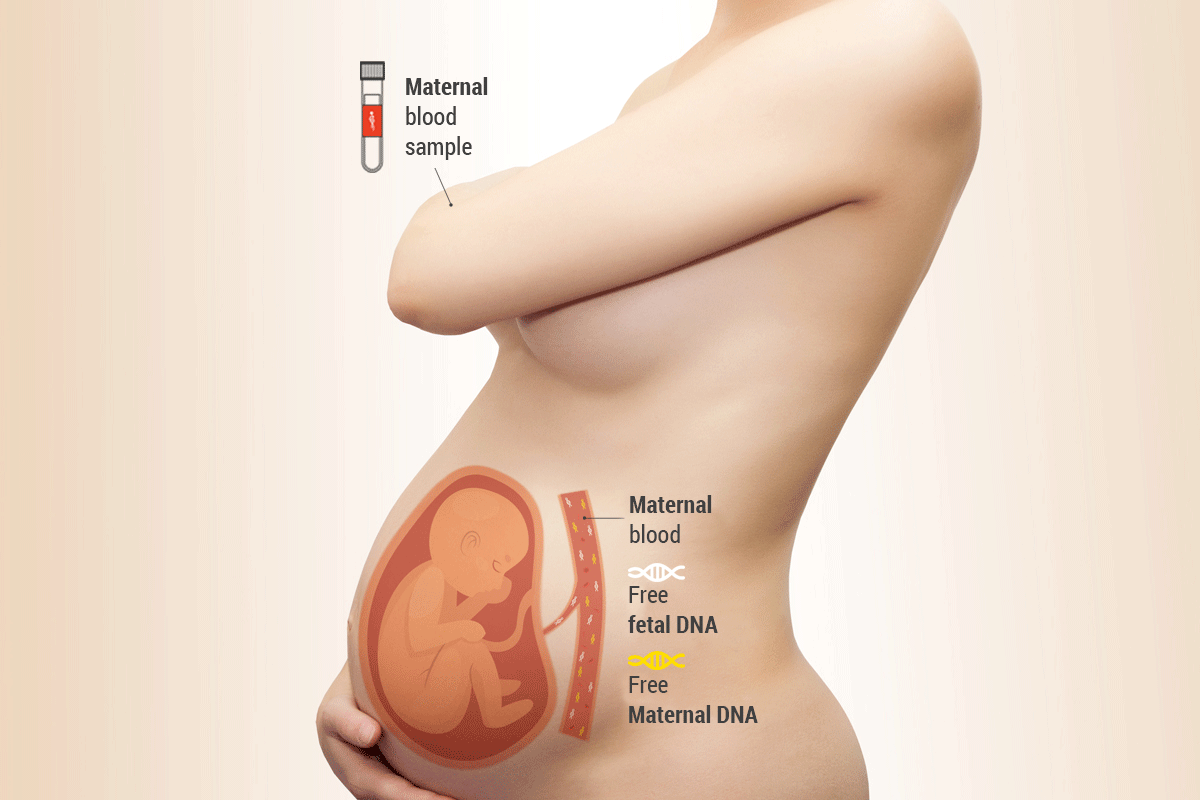
During pregnancy, some fragments of fetal DNA circulate in the maternal blood with a concentration that increases over 9 months, then decreases after delivery. An optimal level for proceeding with the execution of FetalDNA is obtained already after 10 weeks of gestation. At this time, from our clinical validation studies, it is possible to obtain a very high specificity and sensitivity of the examination.
From the 10th week, therefore, it is possible to undergo a simple maternal blood sample, to investigate the presence of any genetic anomalies.