The first group reported 197 women with a positive NIPT test who underwent an invasive prenatal examination (amniocentesis or CVS) to confirm the diagnosis (TAB 1).
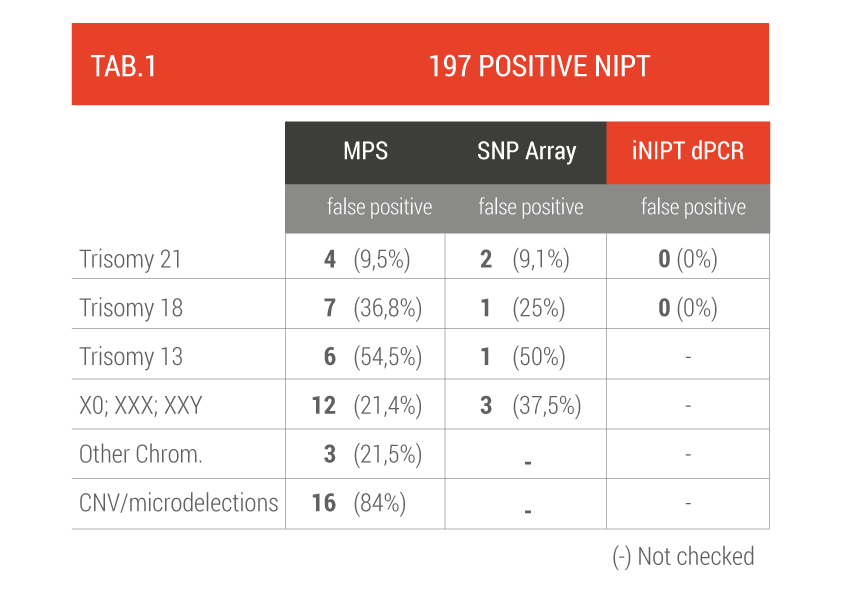
False positives range from 10% to 84% (for microdeletions), 9.5% for T21 and 36.8% for T18, dPCR reduces to zero the false positive rate for fetal abnormalities of chromosome 18 and 21.
Altamedica team performed the FetalDNA test preclinically | iNIPT™ technology to validate its accuracy compared to traditional NIPT.
The second group included 188 women who underwent invasive prenatal diagnosis after a negative NIPT test but there was an ultrasound suspicion of fetal chromosomal pathology (TAB 2).
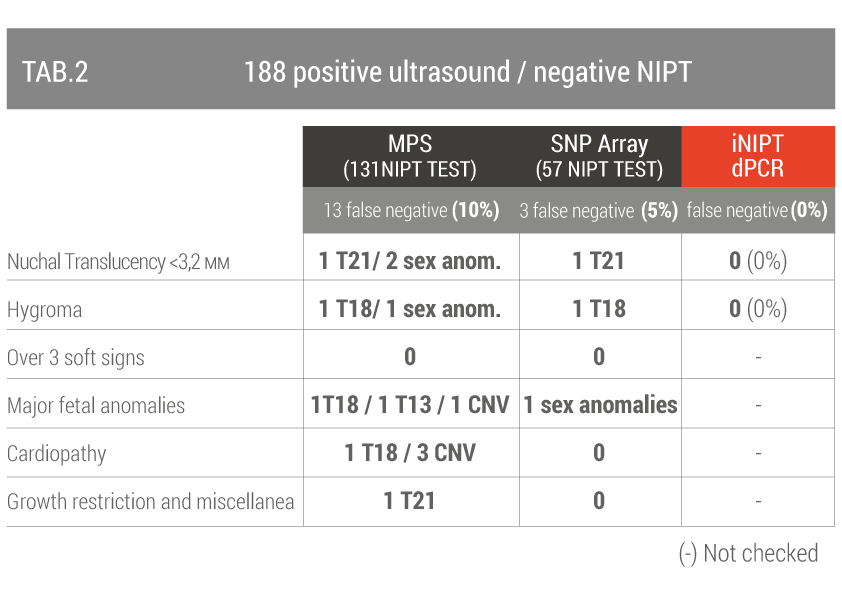
False negatives range from 5% to 10%, dPCR reduces the false negative rate for fetal abnormalities of chromosome 18 and 21 to zero.
The Altamedica team executed the FetalDNA test | iNIPT ™ technology preclinically in order to validate its accuracy compared to traditional NIPT.